Inflammatory Bowel Disease: An Overview
More than one in 200 Americans live with inflammatory bowel disease (IBD). Scientists at BRI are studying IBD from a variety of angles to understand what goes wrong in the gut to trigger this disease and develop new ways to treat, stop and even prevent it.
Scientists in BRI’s innovative Gut Immunity Program are working to better understand immune system cells and processes within the gut. They’re using innovative tools like organoids (tiny replicas of the human gut) to accelerate their progress. Translational scientists at BRI are using knowledge of immune response processes to create therapies that target what has gone awry. We also work closely with the Center for Digestive Health at Virginia Mason Franciscan Health on clinical trials that study the effectiveness and safety of new treatments.
What Is Inflammatory Bowel Disease?
Inflammatory bowel disease (IBD) is the umbrella term for autoimmune disorders that cause ongoing (chronic) inflammation, pain and bleeding in the digestive tract (the series of hollow organs connected to one another that span from the mouth to the anus). Crohn’s disease and ulcerative colitis (UC) are the two most common forms of IBD. Both happen when the immune system mistakenly attacks the gut. The main difference between Crohn’s disease and ulcerative colitis is the location of this swelling and irritation (inflammation).
At least 1.6 million Americans live with inflammatory bowel disease, and the incidence is on the rise. IBD is usually diagnosed between ages 15-35 and 55-70. It affects men and women at about the same rate.
What is Crohn's disease?
Crohn’s disease can affect any of the organs along the digestive tract, though it is most common in the lining of the last part of the small intestine (the ileum) and the beginning of the large intestine.
Crohn’s disease typically causes inflammation in some places, but not others. The symptoms of Crohn’s disease usually develop over time, but sometimes they come on suddenly.
What is Ulcerative Colitis?
Ulcerative colitis (UC) causes inflammation and sores (ulcers) in the large intestine (which includes the colon and the rectum). It affects the innermost layer of the large intestine’s lining (the mucosa). Unlike Crohn’s disease, the inflammation in UC is continuous and not patchy. In most people, symptoms of UC develop over time rather than all at once.
What Causes Inflammatory Bowel Disease?
Inflammatory bowel disease (IBD) happens when the immune system mistakenly begins attacking the digestive tract, but scientists still don’t know exactly why it happens or how it starts. BRI scientists are working to pinpoint its causes and are currently investigating the interplay of genetics, environmental triggers, and why the immune system starts attacking healthy tissue.
What Are Risk Factors for Inflammatory Bowel Disease?
- A close family member like a parent, sibling or child who has inflammatory bowel disease (IBD)
- Age (usually diagnosed before age 30)
- Northern European ancestry (though IBD can occur in any race)
- Environmental factors like living in a northern climate, a developed country or an urban area
- Imbalance in gut microbiome
What is the Difference Between Inflammatory Bowel Disease and Irritable Bowel Syndrome?
Though inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) have some common symptoms – like abdominal pain, constipation and diarrhea – they are not the same condition and require different treatments.
IBS is a group of symptoms that happen when the bowel isn’t working properly. In IBS, there is no inflammation of the tissue and no sign of tissue abnormality.
IBD is an autoimmune disease that causes inflammation in the digestive tract. IBD damages the tissue and causes tissue abnormalities.
IBS is estimated to affect as many as 15% of the United States’ population, making it much more common than IBD, which affects about 1.3% of people in the U.S. In addition, IBS is more common in women than in men, while IBD affects women and men at about the same rate.
What Is the Latest Research Into Inflammatory Bowel Disease?
For three decades, BRI has been working to understand the cells and processes that cause inflammatory bowel disease (IBD). Our research includes:
- Identifying which cells and processes cause IBD.
- Learning why immune cells attack harmless bacteria.
- Understanding the role gut bacteria play in IBD and the immune system overall.
- Finding ways to slow down or tire out the cells that cause IBD.
- Figuring out how to match people to the treatments that will work best for them.
Labs Studying Inflammatory Bowel Disease
Harrison Lab
The Harrison Lab studies the mechanisms controlling host-microbe interactions at barrier tissues, primarily the skin and the gut with the goal to understand how these immune cells promote barrier tissue integrity and repair, and to understand how this goes awry during disease.
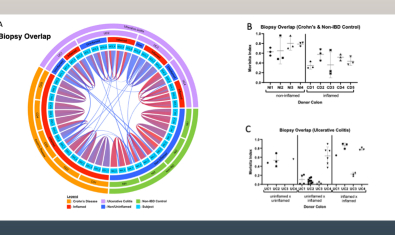
Lord Lab
The Lord lab is investigating how loss of “tolerance” happens in IBD, to learn how the immune system normally coexists peacefully in close proximity to gut contents.
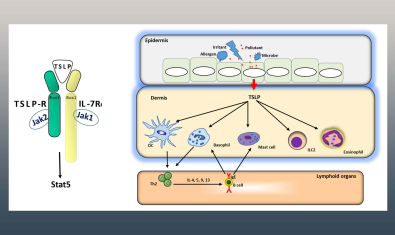
Ziegler Lab
The Ziegler laboratory is investigating the role of the epithelial cytokines TSLP and IL-33 in regulating protective responses at mucosal barrier surfaces such as the respiratory and gastrointestinal tracts.
Clinical Research Studies in Digestive Health
We have ongoing clinical research studies in several areas of digestive health. Studies labeled as “Enrolling” are actively recruiting new participants while studies labeled as “Closed to Enrollment” are still active but no longer seeking new participants.
Please email Digestive Health Research or call (206) 341-1021 for more information.
- Biliary and Liver Disease
A Phase 3 Study to Evaluate the Efficacy and Safety of Pegozafermin in Subjects with Compensated Cirrhosis due to Metabolic Dysfunction-Associated Steatohepatitis (MASH)
Principal Investigator: Asma Siddique, MD
Status: EnrollingALTUS: PERFORMANCE OF A MULTI-TARGET HEPATOCELLULAR CARCINOMA (HCC) TEST IN SUBJECTS WITH INCREASED RISK
Principal Investigator: Asma Siddique, MD
Status: Enrolling
A RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY TO ASSESS THE EFFICACY AND SAFETY OF RIFAXIMIN SOLUBLE SOLID DISPERSION (SSD) TABLETS FOR THE DELAY OF ENCEPHALOPATHY DECOMPENSATION IN CIRRHOSIS (RED-C)
Principal Investigator: Blaire Burman, MD
Status: Closed to Enrollment- Clostridioides difficile (C. diff)
-
Principal Investigator: Gautam Mankaney, MD
Status: Enrolling - Crohn's Disease
-
Principal Investigator: Tim Zisman, MD
Status: Closed to EnrollmentCrohn's Disease: Risankizumab versus Ustekinumab for Subjects with Crohn's Disease
Principal Investigator: Tim Zisman, MD
Status: Closed to EnrollmentA Phase 3, Multicenter, Open-Label, Long-Term Extension Study to Evaluate the Long-Term Efficacy and Safety of Mirikizumab in Patients with Crohn's Disease
Principal Investigator: Tim Zisman, MD
Status: Closed to EnrollmentA Phase 2, Multicenter, Open-Label Extension (OLE) Study to Observe the Long-Term Efficacy, Safety, and Tolerability of Repeated Administration of Upadacitinib (ABT-494) in Subjects with Crohn's Disease
Principal Investigator: Tim Zisman, MD
Status: Closed to EnrollmentA Multicenter, Randomized, Double-Blind, Placebo-Controlled Maintenance and Long-Term Extension Study of the Efficacy and Safety of Upadacitinib (ABT-494) in Subjects with Crohn's Disease who Completed the Studies M14-431 or M14-433
Principal Investigator: Tim Zisman, MD
Status: Closed to Enrollment - Diverticulitis
Protocol for the Comparison of Surgery and Medicine on the Impact of Diverticulitis (COSMID) Trial
Principal Investigator: Val Simianu, MD
Status: Enrolling- Familial Adenomatous Polyposis (FAP)
REC-4881-201 A PHASE 2, MULTICENTER, TRIAL TO EVALUATE THE EFFICACY, SAFETY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF REC-4881 IN PATIENTS WITH FAMILIAL ADENOMATOUS POLYPOSIS (FAP)
Principal Investigator: Gautam Mankaney, MD
Status: Enrolling- Gastroparesis
Examination of Programming with the Enterra® Therapy System in a Double-Blinded, randomized, Prospective Study In the Treatment of Nausea and Vomiting Symptoms using Gastric Electrical Stimulation
Principal Investigator: Pierre Blais, MD
Status: Enrolling- Stricture
Paclitaxel Coated balloon for the Treatment of chronic bEnigN sTricture- Bowel
Principal Investigator: Joanna Law, MD
Status: Enrolling- Ulcerative Colitis
Casting Light on Urgency and Effectiveness of Advanced Therapies in Ulcerative Colitis (CLUES-UC): A Non-interventional, Observational Cohort Study of Mirikizumab and Other Biologics in Adult Participants with Moderately to Severely Active Ulcerative Colitis
Principal Investigator: Tim Zisman, MD
Status: Enrolling
A Multicenter, Randomized, Double-Blind, Placebo-Controlled 52-Week Maintenance and an Open-Label Extension Study of the Efficacy and Safety of Risankizumab in Subjects with Ulcerative Colitis Who Responded to Induction Treatment in M16-067 or M16-065
Principal Investigator: Tim Zisman, MD
Status: Closed to EnrollmentA Phase 2b Randomized, Double-blind, Active- and Placebo-controlled, Parallel-group, Multicenter Study to Evaluate the Efficacy and Safety of Induction and Maintenance Combination Therapy with Guselkumab and Golimumab in Participants with Moderately to Severely Active Ulcerative Colitis
Principal Investigator: Tim Zisman, MD
Status: Closed to EnrollmentA Phase 3 Multicenter, Open-Label Extension (OLE) Study to Evaluate the Long-Term Safety and Efficacy of ABT-494 in Subjects with Ulcerative Colitis (UC)
Principal Investigator: Tim Zisman, MD
Status: Closed to Enrollment