Partner with BRI to build a healthy immune system for everyone
BRI collaborates with other research organizations, providing available technologies and materials, and conducting clinical trials in a broad range of disease areas. BRI works collaboratively with scientists in pharmaceutical and biotechnology companies and life science research institutions throughout the world on immune mediated and other diseases. We welcome the opportunity to explore potential research collaborations with you on the human immune system, including autoimmune diseases, cancer, allergies and asthma.

Join us for BRI Immunology Symposium: New Horizons in Immunity and Disease
Benaroya Research Institute’s first annual Immunology Symposium: New Horizons in Immunity and Disease will be taking place on Tuesday, May 5, from 8:30 a.m. – 5:30 p.m. at Bell Harbor International Conference Center, followed by a reception at BRI. We’re excited to bring together leading voices from the local and national immunology research community for a day of collaboration, learning, discussion and networking.
We hope that you’ll be able to join us.
Opportunities for collaboration using BRI resources
BRI resources offer unique access to samples, innovative tools and expertise to fuel research discoveries.

Research Collaborations Using BRI Biorepositories
BRI actively maintains 11 different biorepositories, including one with healthy control samples for comparison purposes. Scientists use biological samples from the repositories to better understand the biomarkers associated with the progression of the diseases and identify targets for new therapies.
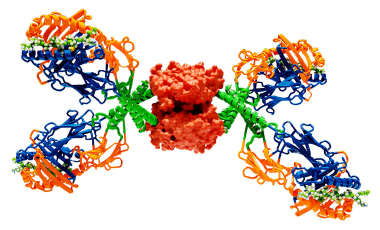
Build a Tetramer
Our Tetramer Core Laboratory provides MHC class II tetramer reagents for collaborators both within and outside BRI. These tetramers are synthetic protein conjugates that allow the direct detection of antigen specific T cells by flow cytometry.

CATA Group
The Cell and Tissue Analysis (CATA) Group at BRI offers an extensive array of flow cytometry, imaging and histology resources for both BRI investigators and the surrounding biotech community.

Human Immunophenotyping Core
The Human Immunophenotyping (HIP) core at the Benaroya Research Institute (BRI) provides access to cutting-edge technologies and services enabling study of human immune-mediated diseases. A full spectrum of services is available, from guidance on study design and training on new technologies to execution, data analysis and interpretation. The HIP core serves investigators from BRI and Virginia Mason Medical Center, as well as other institutions, including both non- and for-profit organizations.
Opportunities for collaboration: Research Areas
From basic science breakthroughs to clinical stage testing, BRI research advances discovery and understanding of the human immune system.
Immunogenicity Determination Of Biologic Drugs
Recombinant protein therapeutics play an increasingly important role in the treatment of human diseases. However, antibody responses to these protein drugs can adversely impact their safety and efficacy. High affinity antibody responses are preceded by CD4+ T cell responses, which control or provoke the undesirable antibody responses. BRI has developed a platform technology that can be utilized to rapidly and systematically identify immunogenic “hot spots” within protein therapeutics, visualize T cell responses against these proteins, and rationally de-immunize their sequences at key amino acid positions.
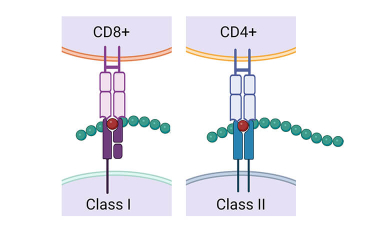
Rapid Epitope Identification
The identification of T-cell epitopes is crucial for the development of effective vaccines against infectious agents and tumors, as well as in the design of Ag-specific modes of immunotherapy for autoimmune and immune-mediated diseases. BRI has developed a tetramer-guided epitope mapping (TGEM) approach to rapidly identify T-cell epitopes. The TGEM approach is based on the direct detection of binding of the TCR to the MHC–peptide complex. It has the advantage of reflecting the dominant T-cell specificity in the test sample, enabling it to be used with peripheral blood from patients or immunized research participants.
Clinical Research at BRI
BRI is currently conducting over 400 clinical studies. Our highly trained research staff have access to a dedicated Clinical Research Center, and most importantly, to a cadre of dedicated physicians at BRI and VMFH. We enroll studies from early stage mechanistic efforts through Phase III clinical trials.

Mechanistic Studies with BRI
We collaborate with industry on a wide range of mechanistic studies. This could include anything from short-course mechanistic/Phase 1-2 studies to probe the immune changes that occur in the context of therapy - to single-cell sequencing of samples from individuals in our biorepository to understand the transcriptional changes and TCRs associated with disease or therapy. We are very interested in understanding what may explain response to therapy across autoimmunity using multiple cutting edge modalities.

Collaborate with BRI Scientists
BRI partners with scientists around the world, bringing our technology, biorepositories and expertise to global research efforts. We form partnerships across all levels of science and areas of research.
Business Development Team
The Business Development team at the Benaroya Research Institute at Virginia Mason Franciscan Health (BRI) oversees BRI’s intellectual property portfolio, research collaborations, technology licensing and material transfers and reviews all related agreements and contracts to facilitate these activities.
For inquiries regarding available technologies for licensing, collaboration opportunities, material transfers or other inquiries, please contact us.

Bolong Cao, PhD, MBA






