Human islet antigen reactive CD4+ memory T cells play a key role in the pathogenesis of autoimmune type 1 diabetes (T1D). Using scRNA-seq, we recently identified a class of autoreactive T cell receptors (TCRs) that share TCR TRA chains between individuals (“public” chains). Public TCRs sharing TRA chains were elevated in new-onset T1D patients.

Public TCR sequences had shorter junction sequences, suggestive of fewer random nucleotide insertions, and hence, were more germline like than private TCRs. Public TRA junctions were often paired with mismatched TCR TRB junctions; remarkably, some of these TCRs exhibited cross-reactivity toward distinct islet antigen peptides.
Our more recent work has involved probing the role of these public TCRs in autoimmune and anti-infectious agent immune responses. We have used TCR sequences as barcodes to determine the extent of infiltration of autoreactive CD4+ T cells from blood into pancreata of non-T1D and T1D organ donors and found extensive sharing at the amino acid level of perfectly matched or single mismatched junctions, comprising >1/3 of unique autoreactive TCRs of multiple specificities. We further show that these pancreas T cell-matching autoreactive CD4+ T cell TRA junctions are largely public sequences, paired with diverse TRB chains, that display sequence features of more germline-like (innate) epitope engagement and accumulate in peripheral blood near the time of clinical diagnosis of T1D.
We are evaluating the TCR binding properties of these pancreas T cell matching autoreactive CD4+ T cell TRA junctions, as well as the timing of their appearance during development of T1D. We also are evaluating how this class of TCRs is linked to outcome of SARS-CoV2 infection in unvaccinated individuals.
Additional Research Projects
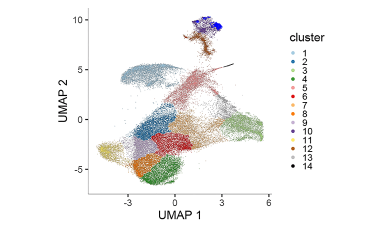
Elucidating immune processes in tumors that affect patient survival and response to therapy
We are investigating whether Immune Checkpoint Inhibitor blockade in cancer treatment promotes the proliferation and survival of T cells specific for self-antigens and tumor antigens that ultimately contribute to adverse immune events or response to therapy.
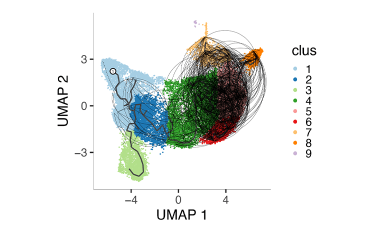
Exhausted CD8+ T cell populations linked to beneficial response in T1D
Using next generation sequencing approaches we are investigating the T cell ancestry and phenotypes of exhausted CD8+ T cell populations that are associated with beneficial response to T cell depleting agents in T1D.


