Over the last decade, we have witnessed a major advance in immunotherapy for human cancer patients with immune checkpoint inhibitor (ICI) treatment. An unfortunate side effect of these therapies is the development of immune-related adverse events (irAE). Currently, it is not known whether development of irAE can be uncoupled from successful cancer therapy.
To develop molecular markers of irAE development, we are investigating antigen-specific T cells in ICI-treated cancer patients, in collaboration with Drs. Erik Wambre and Jane Buckner at BRI, Dr. JP Flores at Virginia Mason Medical Center, and Dr. Petros Grivas at the Fred Hutchinson Cancer Research Center. The central hypothesis for this project is that ICI blockade promotes the proliferation and survival of T cells specific for self-antigens that ultimately contributing to adverse immune events.

Our objective is to determine if expansion of autoreactive cells predicts development of autoimmunity and/or efficacy of ICI therapy. We are recruiting cancer patients who are receiving ICI therapy and testing for linkage between the frequency and phenotypes of tumor and autoimmune antigen reactive cells in patient samples collected before and after treatment. An example of our approach is shown in the above figure, where we have profiled CD8+ T cells recognizing Tumor Associated Antigens (TAA) by scRNA-seq before and after ICI therapy. We are investigating how these TAA-specific CD8+ T cells are linked to response to therapy and development of irAEs.
Additional Research Projects
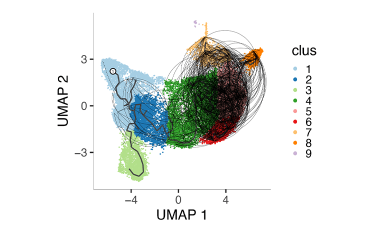
Exhausted CD8+ T cell populations linked to beneficial response in T1D
Using next generation sequencing approaches we are investigating the T cell ancestry and phenotypes of exhausted CD8+ T cell populations that are associated with beneficial response to T cell depleting agents in T1D.
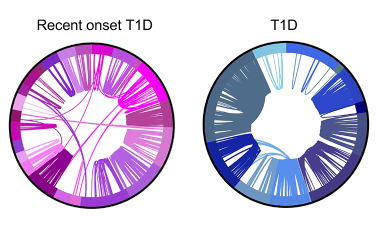
Shared germline-like TCR alpha chains in autoreactive CD4+ T cells in T1D
We found a class of autoreactive T cell receptors (TCRs) that share TCR TRA chains between individuals and that these TCR TRAs are elevated in new-onset T1D patients. We are investigating the role of these public TCRs in autoimmune and anti-infectious agent immune responses.


