Regulatory T cells (Tregs) defined by expression of CD4, CD25 and the transcription factor FOXP3 play an essential role in maintaining immune tolerance and preventing the development of autoimmunity by suppressing effector T cell (Teff) proliferation and cytokine production. The Buckner lab has been studying Tregs for more than a decade and have made several important contributions to our understanding of human Tregs including the de novo generation of human antigen-specific CD4+CD25+ Tregs, development of in vitro assays for the study of human Treg suppression and the demonstration that Teff resistance to Treg suppression in type 1 diabetes is due to a defect in Teff not in Tregs. In addition, Dr. Buckner has also been involved in a number of clinical trials targeting Tregs, including a phase 1 trial assessing the safety of Treg adoptive immunotherapy in type 1 diabetes.
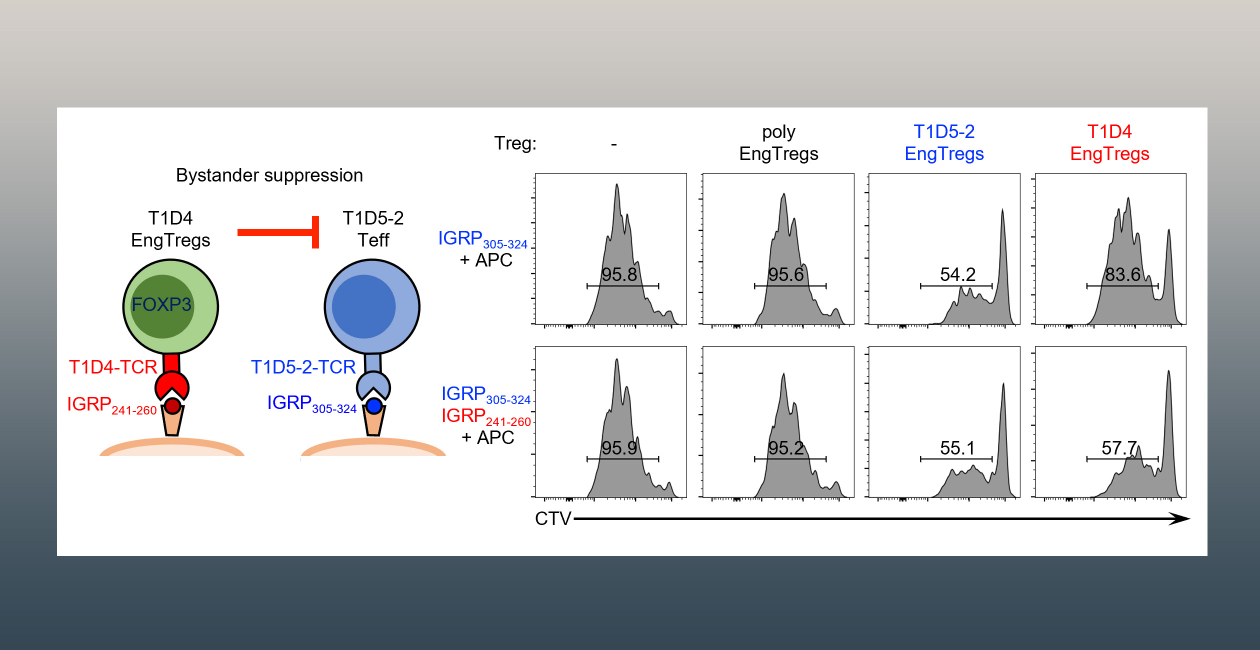
In collaboration with Dr. David Rawlings at Seattle Children’s Research Institute and Dr. Soo Jung Yang at BRI, the Buckner lab is currently focused on developing a Treg-based therapies to suppress the autoimmune response in type 1 diabetes. Our approach involves combining FOXP3 homology-directed repair editing and lentiviral T cell receptor delivery to generate FOXP3 stabilized islet-specific engineered Tregs from primary human CD4 T cells. These islet-specific engineered Tregs are functional and are able to suppress antigen-specific and bystander Teff proliferation and cytokine production in in vitro assays. Notably, they prevent development of diabetes in mouse models of type 1 diabetes. Based on this work, Drs. Buckner and Rawlings founded GentiBio, a biotech company developing engineered Tregs to treat patients with autoimmune diseases, alloimmune inflammatory diseases and allergic diseases. The Buckner lab continues to focus on TCR identification and characterization and is extending this work to other antigens and alternative HLA alleles as well as expanding to other autoimmune diseases such as rheumatoid arthritis.
Together with Dr. Rawlings at SCRI and Dr. Eddie James at BRI, we are also developing engineered Tregs to home to the islet and suppress inflammation and beta cell stress, a hallmark of T1D. This project involves identification and characterization of TCRS targeting neoepitopes generated during beta cell stress, engineering of antigen-specific T cells and also includes developing engineered Tregs with the ability to co-deliver a therapeutic cargo with or without TCR engagement.
Lastly, the Buckner lab is investigating the mechanisms underlying both Treg-mediated suppression and Teff resistance to suppression in the setting of autoimmune disease. We are examining how the cell source, HLA restriction and functional avidity of a TCR influences the suppressive capacity of Tregs. In addition, we are assessing the mechanisms that alter the responsiveness of effector T cells to Treg and how immunotherapies may influence Teff resistance.
Additional Research Projects
Altered T cell function in human autoimmune disease
The Buckner lab has a long-standing interest in understanding how T cell function is altered in human autoimmune disease.
Antigen-specific T cells in autoimmunity
Antigen-specific T cells play a central role in the adaptive immune response, driving the cellular response to foreign antigens and maintaining immune tolerance to self-antigens as well as contributing to the formation of immunological memory.