Antigen-specific T cells play a central role in the adaptive immune response, driving the cellular response to foreign antigens and maintaining immune tolerance to self-antigens as well as contributing to the formation of immunological memory. Antigen-specific T cells are also important in disease settings including autoimmune disease and cancer. T cells recognizing self-antigens have been implicated in the pathogenesis of several human autoimmune diseases including rheumatoid arthritis (RA), multiple sclerosis (MS) and type 1 diabetes (T1D), and tumor-specific T cells are critical for anti-tumor immunity. Despite this knowledge, there remains substantial gaps in our understanding of how antigen-specific T cells contribute to disease, including which antigens drive disease and how T cells recognizing these antigens are triggered to become pathogenic. Ultimately, a full understanding of antigen specific T cell fate and function in health and disease will allow development of therapies to treat and prevent autoimmune diseases.
The Buckner lab is working to understand the role of antigen-specific CD4+ T cells in the pathogenesis of rheumatoid arthritis, and also as a biomarker of disease activity and/or response to therapy. Together with Dr. Eddie James at BRI, we are focused on identifying and characterizing the frequency and phenotype of CD4+ T cells that are specific for citrullinated antigens. For these studies, we analyze biological samples from cross-sectional and longitudinal cohorts of patients with RA utilizing cutting edge tools including multiplex tetramer approaches and single cell profiling. We are also evaluating antigen-specific T cell responses with respect to T cell receptor repertoire and transcriptional profile. Our previous studies have shown that citrullinated antigen-specific CD4+ T cells are increased in patients with RA compared to healthy control subjects and their frequency is influenced by disease duration and therapy. We have also identified a number of novel citrullinated epitopes including several from the extracellular matrix protein tenascin-C. These tenascin-C epitopes have the unique characteristic of promoting responses in both CD4 T cells and B cells, suggesting a mechanism by which autoimmunity is amplified and could promote progression to RA. The search for pathogenic antigen-specific T cells in RA is an ongoing project in the Buckner and James Labs and has recently expanded with a collaboration with Dr. Bill Kwok at BRI. This project is focused on identifying antigen-specific T cells in synovial tissue, the site of inflammation in RA, and is also expanding to other post-translationally modifications besides citrullination. Lastly, the Buckner lab collaborates with Drs. Michael Holers and Kevin Deane at the University of Colorado investigating antigen-specific CD4+ T cells in individuals at risk for the development of RA.
The Buckner lab is also investigating antigen-specific CD4+ T cells in the setting of cancer, in collaboration with Drs. Peter Linsley and Erik Wambre at BRI. The central hypothesis for this project is that PD-1 blockade promotes the proliferation and survival of CD4 T cells specific for self-antigens ultimately contributing to adverse immune events. Our objective is to determine if an increase in the frequency of autoreactive cells, and their specificity predicts development of autoimmunity in the context of checkpoint inhibitor treatment. Further, we are investigating if there is a link between the expansion and features of autoreactive CD4 T cells and anti-tumor and autoimmune responses. To do this, we are collaborating with Dr. JP Flores at Virginia Mason Medical Center, Dr. Petros Grivas at the Fred Hutchinson Cancer Center, Dr. Nimrata Singh at the University of Washington and Dr. Sang Kim from MD Anderson Cancer Study who are recruiting patients with cancer that are receiving immune checkpoint inhibitors, either at the time they develop an autoimmune complication or for a longitudinal cohort with samples collected before and after therapy.
Additional Research Projects
Altered T cell function in human autoimmune disease
The Buckner lab has a long-standing interest in understanding how T cell function is altered in human autoimmune disease.

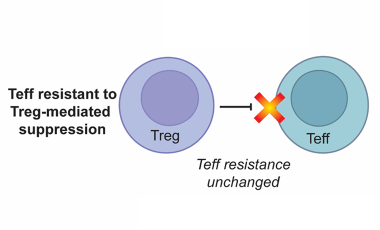
Regulatory T cells in the context of autoimmune disease
Regulatory T cells (Tregs) defined by expression of CD4, CD25 and the transcription factor FOXP3 play an essential role in maintaining immune tolerance and preventing the development of autoimmunity by suppressing effector T cell (Teff) proliferation and cytokine production.



