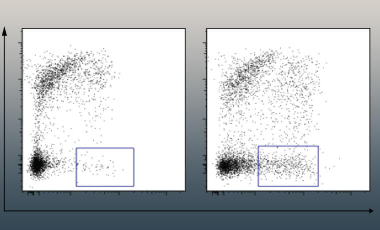
Upon activation, naïve T cells proliferate, differentiate and ultimately adopt a number of distinct fates, including establishment as long-lived tissue resident memory T cells. These tissue resident T cells can act as sentinels of the immune system, able to rapidly respond to tissue injury and reinfection. As yet, how these cells may lay dormant for years, and yet react within minutes of injury remains poorly understood.
We are working to understand how tissue resident T cells adopt a poised transcriptional and translational profile that allows rapid and tailored responses to tissue injury, whilst maintaining tight regulation over these potent and toxic effector molecules during homeostasis.
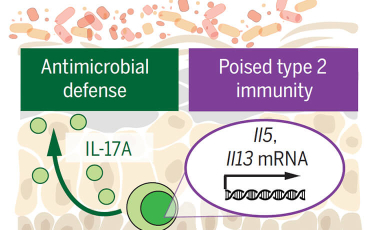
The Harrison lab is working to further understand the post-transcriptional mechanisms that allow storage and stability of mRNA for effector cytokines, and the cues that trigger rapid protein translation and release to promote wound healing and tissue repair. The lab is interested in understanding how dysregulation of these mechanisms may result in aberrant tissue repair or fibrosis in inflammatory tissue disorders.