Autoimmune type 1 diabetes is considered a T cell mediated disease in which autoreactive CD4 and CD8 effector T cells destroy insulin-producing β-cells located in pancreatic islets, leading to life-long dependence on exogenous insulin. Surprisingly, islet reactive CD4 effector T cells can also be found in the blood of non-diabetic individuals.
This suggests that autoreactive effector T cells from T1D subjects are more pathogenic than healthy controls or that islet reactive regulatory T cells in T1D patients fail to suppress the function of effector cells. To address this question, the Cerosaletti lab is studying islet reactive CD4 effector and regulatory T cells using high dimensional flow cytometry coupled with single cell RNA sequencing to capture their gene expression profiles and T cell receptor sequences. This approach is coupled with technology to determine what islet proteins are recognized by individual islet reactive T cells.
These studies have led to the identification of expanded clones of islet reactive CD4 effector T cells with innate-like features that are expanded at the time of disease onset. The lab is currently investigating whether these innate-like islet reactive T cells are linked to disease progression.
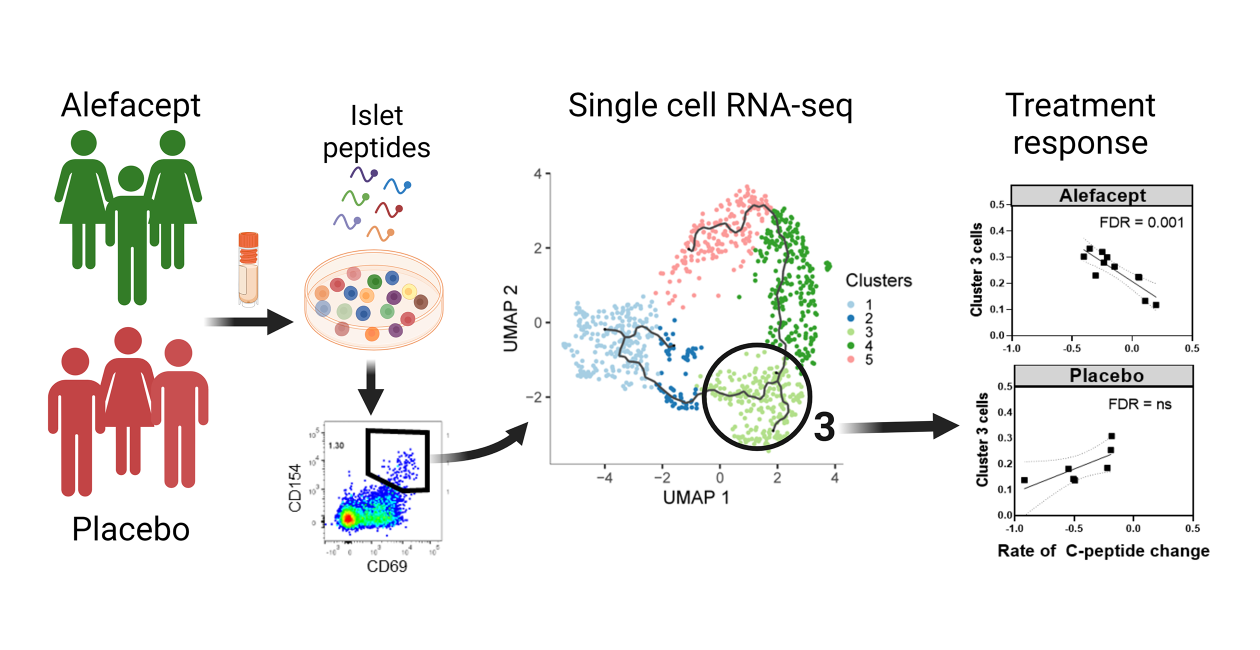
We have also identified a unique subset of proinflammatory islet reactive CD4 effector T cells at disease onset that is linked to the response to immunotherapy in a T1D clinical trial.
In parallel, the lab is studying the gene expression profile, T cell receptor repertoire and specificity of islet reactive regulatory T cells in T1D patients compared with non-diabetic donors to understand how these cells differ in T1D.
Additional Research Projects
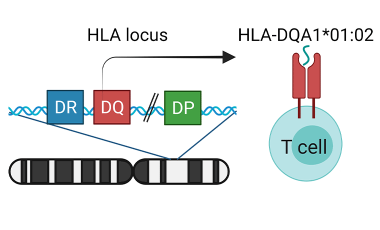
Genetics of response to allergy immunotherapy
The Cerosaletti Lab is studying the role of genetic variation in the HLA class II genes in the response to peanut oral immunotherapy by analysis of antigen presentation and recognition by peanut specific T cells.
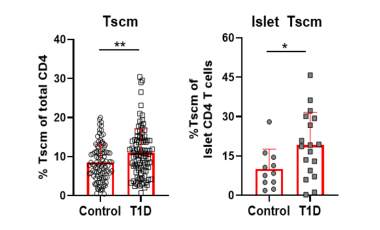
Stem-like memory CD4 T cells in type 1 diabetes
The Cerosaletti lab is studying stem-like memory CD4 T cells (Tscm) in T1D to understand the mechanism leading to expansion of these cells in T1D and determine if Tscm levels influence disease progression and response to therapy.