Testing a New MS Therapy
Last winter, Elena Connors commuted over treacherous roads from Richland to Walla Walla for a job as a Russian language interpreter. She blamed the strenuous trips for her painful headaches and put off seeing a doctor until they were so distressful, her husband, Charlie, took her to the emergency room.
“I had a series of tests, and around midnight the doctor announced that I likely had multiple sclerosis (MS),” says Elena. “We were surprised. But looking back, I realize that my body was changing over time. If the weather was hot, my energy level was so low that I did my shopping before it was unbearable. Now I realize that the fatigue was due to MS.”
Upon her diagnosis, a relative “suggested seeing one of the wonderful doctors at Virginia Mason and honestly, it was the best decision ever!” says Elena. Though dealing with the disease was difficult for her and her family, Elena kept a positive outlook. “It took me a couple of months to realize that in an active stage of MS, I couldn’t push myself very hard,” she notes. “I was taking a medicine which helped, but I couldn’t tolerate the side effects very well and it limited me from doing things.”
Her physician, Virginia Mason neurologist and clinical researcher Lucas McCarthy, MD, informed her about a new research study that is testing a drug with potentially fewer side effects than Elena’s current medicine. “I was interested in helping get the new medicine approved to help me and to give other patients one more choice to lead a quality life,” says Elena. “If scientists can improve existing medications or develop better ones, I feel it’s important to be part of the research.
“I joined the trial and my family noticed my almost sudden improvement within a couple of weeks. I had more energy, a better mood and I’m in a better place overall. I look forward to my visits to Virginia Mason. My wonderful team includes Dr. McCarthy, Katherine Wilder and Evelyn Fox. I feel so confident discussing any issues with them.”
The goal of the study was to see if an experimental oral drug, similar to a currently available MS medication, Tecfidera, will be just as effective as with fewer side effects. “Tecfidera is very safe and effective for prevention of MS relapses, but it has significant gastrointestinal side effects that lead to its discontinuation in a fair number of patients,” says Dr. McCarthy, principal investigator for the study at Virginia Mason and Benaroya Research Institute at Virginia Mason (BRI). “We would like to find a drug as effective that is more tolerable.”
In the initial study, half the research participants took this experimental drug and half took Tecfidera. Participants who finished the five-week study could then enroll in a two-year study of the experimental drug, which Elena chose to join.
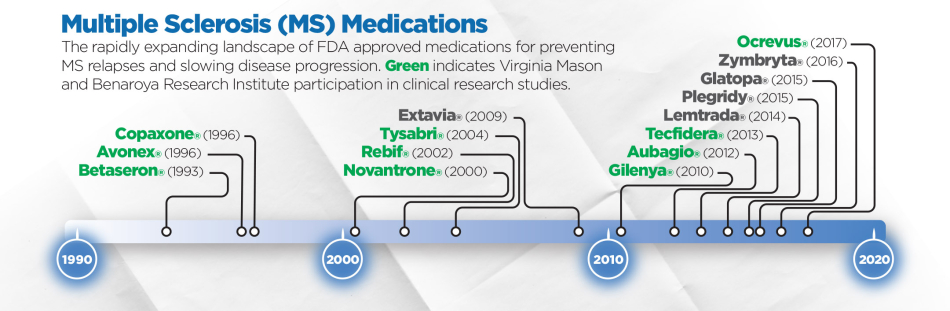
“There are now more than 15 FDA approved medications for stabilizing MS, with more than eight approved just since 2010,” says Dr. McCarthy. “The field of MS treatment is rapidly evolving with more effective therapies, but some options also have more serious risks and side effects. Despite these advancements, there are still many more unmet needs in MS treatment, with some patients and types of MS, such as progressive MS, that have yet to benefit from existing approved therapies.
“Clinical trials give our patients the opportunity to access a wide array of cutting-edge therapies with the goals of having more tolerability and efficacy at stopping MS activity and improving their quality of life,” emphasizes Dr. McCarthy. “Participating in clinical trials, including our observational, non-interventional trials, lets our patients help advance knowledge about this complex disease.”
“Research helped me find a new possible long-term medication once it’s approved by the FDA,” says Elena. “It gave me hope to lead the quality of life I used to enjoy before. When we worry about something we can’t control, we lose confidence in ourselves, the gloomy prognosis can take over and we bury ourselves in negative thoughts making our health worsen. I hope more people can consider clinical trials for new medications to make a difference in their lives. It’s great that VM and BRI can provide access to trial medications for lots of people!”
About Multiple Sclerosis
Multiple sclerosis (MS) is an autoimmune disease in which the body’s immune system mistakenly attacks myelin, the fatty substance that surrounds and protects the nerve fibers in the central nervous system (brain and spinal cord). When the myelin is damaged, the nerve impulses are not transmitted as quickly or efficiently, resulting in symptoms such as numbness in the limbs, fatigue, dizziness, paralysis and/or loss of vision. Symptoms of MS will often improve and relapse with time and vary from one person to another. In progressive forms of multiple sclerosis, they gradually worsen.
Immuno-what? Hear the latest from BRI
Keep up to date on our latest research, new clinical trials and exciting publications.


